Objectives: To further develop the access and availability of the comprehensive high quality of care in Comprehensive Cancer Care Networks to all European member states and align the high standards in cancer care for all quality assured institutions with a focus on the interfaces between care and research (CCCNs) and Comprehensive Cancer Centres (CCC).
Description: CCCNs together with CCCs are the foundation to reach the goal of Europe‘s Beating Cancer Plan (EBCP) to ensure high standards in cancer care and delivering higher quality care. In the previous Joint Action CancerControl (CanCon) and Joint Action Innovative Partnership for Action Against Cancer (JA iPAAC) Work Package10. CCCNs where defined and successfully piloted with the aim to ensure higher-quality care and reducing inequalities across the European Union (EU). WP6 is therefore a continuation of the theoretical framework of CCCNs developed during the JA CanCon and its translation into practice which was successfully achieved JA iPAAC.
WP6 with representatives from 35 member organisations from 19 member states developed and piloted recommendations using the example of Lung Cancer CCCNs in order to establish and expand access to quality-assured oncological care in all Member States (MS). In detail, this means practical instruments have been developed to govern oncological care successfully (e.g., methodology to develop tumour and stage specific patient pathways, Quality Indicators (QI) for Lung Cancer, Set of Standards for CCCNs (generic and tumour-specific), training manual for future CCCNs as well as an updated certification framework for the implementation of CCCNs. The developed instruments were field tested and two pilot sites in Poland and Luxembourg set up Lung Cancer CCCNs which were successfully peer-reviewed.
Overview of tasks
Task 6.1: Definition of Comprehensive Cancer Care Networks (CCCN) and its interfaces with Comprehensive Cancer Centres (CCC)
Task 6.2: Development of support instruments to set-up CCCN in different member states
Task 6.3: Development of Set of Standards and Quality Indicators for Lung Cancer Care
Task 6.4: Enhancing patient centeredness in CCCNs with patient pathways
Task 6.5: Feasibility study: Setting up certified Lung Cancer CCCNs and implementing of results from task 2-4 in two pilot-sites
Work Package Meetings
Outputs and deliverables
Task 6.1 Definition of Comprehensive Cancer Care Networks (CCCN) and its interfaces with Comprehensive Cancer Centres (CCC)
Description: Definition of the role of CCCNs within the landscape of national governance of oncological care with a focus on the interfaces with CCC (joint task with WP8)
The goal of task 6.1. is an updated definition on CCCNs and their role within the landscape of national governance of oncological care with a focus on the interfaces with CCCs.
Sub-task 6.1.1 Mapping of organisational models of networks built around a CCC and CCCNs
Sub-task 6.1.1 is a joint task with WP8. WP8 conducted a mapping of organizational models of networks built around CCCs and CCCNs. The results of the mapping of organizational model can be found here.
Sub-task 6.1.2 Definition of interfaces between research (CCC) and care (CCCN) structures
Sub-task 6.1.2 focused on the interfaces between research (CCC) and care (CCCN) structures with the goal to define and describe the interfaces between CCCN and CCCs as well as to update the CCCN definition developed in JA CanCon.
Report on CCCN definition and interfaces
Download Document (PDF file, 511 kB)
Task 6.2. Development of support instruments to set-up CCCN in different member states
Description: Development of a training concept with instruments to enable and empower member states to set up quality assured CCCNs.
The goal of task 6.2 is to support the implementation and certification of CCCNs through the development of a training concept with support instruments to enable and empower member states and oncology hospitals/networks to set up quality assured CCCNs.
Sub-task 6.2.1 Baseline assessment
In order to better understand the experiences and challenges potential CCCNs are facing when undergoing the setting up and certification process, task 6.2 started with a baseline assessment.
Download Document (PDF file, 743 kB)
Sub-task 6.2.2 Training Concept
Based on the derived recommendation of the baseline assessment, the training concept was developed to support the setting up of CCCNs consisting of training manual for setting up CCCNs, modular approach and a coaching concept for supporting the set-up CCCNs.
Report on developed Training Concept for set-up of a CCCNs
Download Document (PDF file, 2.027 kB)
Task 6.3. Development of Set of Standards and Quality Indicators for Lung Cancer Care
Description: Apply the instruments developed under JA iPAAC to other tumour entities and derive a Set of Quality Indicators (QIs) for Lung Cancer and develop a Set of Standard (SoS) for Lung Cancer CCCNs.
Sub-Task 6.3.1 Set of Standards for Lung Cancer Care
Sub-task 6.3.1 The Set of Standards depicts all requirements for structure, process and quality standards that need to be full filled to become a certified Lung Cancer CCCN.
The Set of Standards for Lung Cancer has the same structure and table of content as the SoSs developed for colorectal and pancreatic cancer care in the JA iPAAC. In addition, for the cross-sectional disciplines radio-oncology and pathology, separate SoS were developed as these partners often treat multiple tumour entities.
Set of Standards for Lung Cancer Care
Download Document Set of Standards for Lung Cancer Care (PDF file, 581 kB)
Download DocumentSet of Standard for Pathology (PDF file, 252 kB)
Download DocumentSet of Standard for Radio-Oncology (PDF file, 325 kB)
Sub-Task 6.3.2 Development of Set of Quality Indicators
Quality of care is made transparent through QI, which can be used for a continuous quality improvement process. Based on the methodology developed in iPAAC how to define Quality Indicators a set of QI for Lung Cancer was defined. The Set of QIs were checked for feasibility in the WP6 pilot sites.
Updated Methodology for defining quality indicators (QI) in order to monitor and improve oncological care within Comprehensive Cancer Care Networks (CCCNs) – The Development of QI-Sets in Oncology Tool (QISO)
Based on the “The iPAAC Evaluation Tool for QIs in Oncology (iET-QIs)” the methodology to define QI was updated and further developed adding a re-evaluation and updating process for defined QI- that can be used for the monitoring of the quality of care in oncology, for instance in CCCNs. In addition, the updated document documents the process for defining the QI-set for Lung Cancer.
Development of QI-Sets in Oncology Tool (QISO)
Download Document(PDF file, 301 kB)
Final Set of Quality Indicators for Lung Cancer
Download Document(PDF file, 309 kB)
Sub-Task 6.3.2 Pilot study
Automated calculation of quality indicators from hospital data, to analyze guideline adherence in a pancreatic cancer network. This feasibility study tested if Quality Indicators for Pancreatic Cancer can be measured based on automated data extraction from routinely-collected hospital data, thus exploring the way for scalable, unambiguous QI monitoring and value-based healthcare.
Download Document(PDF file, 1.532 kB)
Task 6.4. Enhancing patient-centeredness in CCCNs with patient pathways
Description: To advance patient-centeredness in Comprehensive Cancer Care Networks (CCCNs) and Comprehensive Cancer Centres (CCCs), the methodology developed in the former Joint Action iPAAC in WP10 was enhanced and applied to create a patient pathway that stronger includes the patient voice and patient-centeredness elements. In addition, interoperability guidelines for the digitalization of patient pathways in CCCNs and CCC were prepared.
Sub-Task 6.4.1 Systematic Review and Survey on Patient-Centeredness in CCCNs
The primary goal was to establish a consensus among working group participants regarding the definition and dimensions of patient-centeredness in CCCNs. The results were achieved using a systematic meta-review and a WP6 survey, resulting in a unified definition and model for patient-centeredness in CCCNs. Additionally, an extensive list of patient-centered activities was generated to support the practical implementation of the established model.
Definition of patient-centeredness in CCCNs:
“Patient-centeredness in a Comprehensive Cancer Care Network (CCCN) is a philosophy of care prioritising cancer patients' physical, emotional, and social needs, as well as personal values on every step of the patient pathway. In patient-centered CCCNs, patients are empowered and engaged to become active partners in healthcare in relation to their individual preferences and capabilities with the goal of providing personalised, high-quality, holistic care with the best possible outcomes.”
Model of patient-centeredness in CCCNs: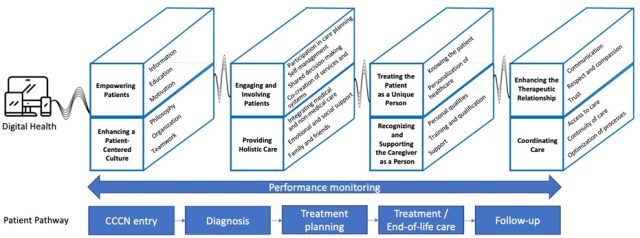
Furthermore, the iPa2-Guide – a methodology for patient pathway development and implementation designed in iPAAC WP10 – was enhanced to include a stronger focus on patient-centered practices. The obtained results (definition, model, and patient-centered activities) were utilized to formulate a set of design principles for patient-centered pathways. The design principles, were then utilised to formulate new patient-centered practices for incorporation into the iPAAC method, aligning with each phase of the pathway lifecycle. An initial evaluation involving five experts from European Cancer Care Organizations yielded promising results, supporting the ongoing development of these patient-centered practices.
Documents and References:
- Report on Task 6.4.1 (PDF file, 374 kB) including the description of the elements of the model of patient-centeredness in CCCNs, the extensive list of patient-centered activities, and the extension of the iPa2-Guide to enhance patient-centeredness during patient pathway development and implementation.
- Hickmann E, Richter P, Schlieter H, Cemazar M, Dudek-Godeau D, Grapentin N, Griesshammer E, Jelenc M, Liutkauskiene S, Ravaud A, Troussard X, Wesselmann S (2024): Operationalizing Patient-Centered Care: A Conceptual Framework for Comprehensive Cancer Care Networks. Submitted to JMIR Cancer, available as JMIR preprint 19/04/2024:59683, DOI: 10.2196/preprints.59683, URL: https://preprints.jmir.org/preprint/59683
Sub-Task 6.4.2 - Development and Agreement of Lung Cancer Patient Pathway Template for CCCNs
A comprehensive lung cancer patient pathway template designed for the application in Comprehensive Cancer Care Networks (CCCNs) was systematically developed using and enhancing the pathway design method established in the previous Joint Action iPAAC (iPa2-Guide, also see Sub-Task 6.4.1 ), with a focus on increasing patient-centredness. For the definition of patient pathways, we refer to the definition agreed upon in iPAAC WP10.
The development process relied on current evidence for lung cancer and included numerous workshops and feedback loops with lung cancer care specialists. To ensure high-quality outcomes and incorporate the patient perspective, patient representatives and their associations were actively involved throughout the process.
The overall lung cancer patient pathway template for CCCNs encompasses the following phases of the patient journey:
- CCCN entry
- staging diagnostics
- treatment planning
- treatment
- follow-up/end-of-life care
- conclusion of CCCN care
Major activities such as "patient consultation," "staging diagnostics," "standard tumour board (TB) meeting," "molecular tumour board (MTB) meeting," and "treatment" are elaborated in separate sub-pathway models. Additionally, quality indicators (reference to Sub-Task 6.3.2 and document “Final Set of Quality Indicators for Lung Cancer”) are integrated along the pathway elements.
To further enhance patient-centeredness within CCCNs using the lung cancer patient pathway template, a comprehensive toolbox of patient-centered practices for each phase of the pathway is provided. These practices are specifically designed to ensure that the patient pathway is genuinely focused on the patient's needs, thereby supporting CCCNs in delivering truly patient-centered care effectively.
Although developed for the context of CCCNs, the pathway's generic nature makes it equally applicable in Comprehensive Cancer Centres (CCCs).
Documents:
- Template for tumour-specific patient pathways: example of lung cancer
Download Document (PDF file, 2.767 kB) - Report on Task 6.4.2 describing the lung cancer patient pathway templated accompanied with patient-centred practices.
Download Document (PDF file, 3.615 kB)
Sub-Task 6.4.3 Patient Pathway Adaptation and Implementation Support in Pilot CCCNs
The lung cancer patient pathway template was adapted by the pilot sites in Luxembourg and Poland to the national, regional and local specifies. These especially include the following types of adaptations:
- Reference to national guideline recommendations
- Detailing the actors involved in the steps and phases along the patient pathway
- Integration of national/regional databases such as cancer registries
- Integration of national requirements such as timelines/ maximum waiting times for diagnostics and beginning of treatment
- Inclusion of the “patient voice” – assessed in patient working groups – along the patient pathway
Download Document (PDF file, 342 kB)
Sub-Task 6.4.4 Preparations for Standardised Digital Implementation of Patient Pathways in CCCNs
The CraNE CCCN Pathway Implementation Guide (IG) supports standardizing cancer care pathways across EU Member States to improve patient outcomes and transnational cooperation. The IG’s core objective is to provide a digital, standardized approach to implementing patient pathways, specifically focusing on lung cancer. The IG aims to enhance the quality and effectiveness of cancer treatment across Europe by leveraging digital tools and standardized health data exchange protocols.
Initially, the patient pathway was modeled using BPMN (see Sub-Task 6.4.2), a visual tool that helps describe roles, events, and data flows in cancer care. While BPMN offers a user-friendly approach, it has limitations in ensuring interoperability, especially in complex healthcare settings. To address this, the IG uses HL7 FHIR, a modern standard recommended by the European Commission for exchanging electronic health data.
The IG uses specific FHIR profiles to represent various elements of the cancer care process, such as activities, diagnostic measures and quality indicators, improving semantic interoperability through standardized medical terminologies like SNOMED CT and ICD.
The IG provides a basis for the expansion and further development of the lung cancer patient pathway. The quality and interoperability can be increased by enriching the patient pathway with further details on the treatment of lung cancer.
Report on Patient Pathways for Lung Cancer Patients
Download Document (PDF file, 6.263 kB)
Task 6.5. Setting up certified Lung Cancer CCCNs
Description: Implementation and roll out of two certified Lung Cancer CCCNs in two Member States. Deliverables from task 6.2-6.4 for Lung Cancer will be implemented in two pilot CCCNs and afterwards peer-reviewed accordingly to the European Framework for the certification of CCCNs.
The goal of task 6.5 is to set up two certified Lung Cancer CCCNs (pilots sites) in two MS and to implement the developed documents from task 6.2.-task 6.4 and to update the European Framework for the certification of CCCNs, which describes the certification process.
Sub-Task 6.5.1 Identification of pilot CCCNs
The following networks agreed to join WP6 as pilot sites:
- Lower Silesian Oncology, Pulmonology and Hematology Center (Wroclaw, Poland)
- Lung Cancer Network Luxembourg
Sub-Task 6.5.2 Updating of certification framework
Based on the document “European Framework for the certification of CCCNs in the course of iPACC”, the evaluation framework was further developed and updated including now a modular certification process (see also task 2 training concept) to allow a step-by-step certification process for CCCNs and to encourage continuous further development.
Download Document (PDF file, 552 kB)
Sub-Task 6.5.3. Implementation of results of task 6.2.-6.4 and Peer Review and certification of CCCN
The pilot CCCNs implement the deliverables from task 6.2 – 6.4 (i.e. Set of Standards for Lung Cancer Care, Set of Standard for Pathology, Set of Standard for Radio-oncology, Set of Quality Indicators for Lung Cancer, training manual for setting up CCCNs, Patient Pathway for Lung Cancer Patients). The Set of Standards for Lung Cancer and the set of QI for Lung Cancer were made available to the two pilot CCCNs.
Both pilot CCCNs were successfully peer reviewed by independent auditors according to the updated Certification Framework for CCCNs.
The auditors congratulated the two pilots CCCNs on their excellent and high performance. The awarding ceremony took place on 1 July 2024 in the scope of the 4th WP6 meeting in Warsaw at the National Institute of Public Health National Institute of Hygiene- National Research Institute.
Congratulations to both pilot CCCNs for their outstanding performance!
-
- Certificate from pilot site Lower Silesian Oncology, Pulmonology and Hematology Center (Wroclaw, Poland).
Download Document (PDF file, 184 kB)
- Certificate from pilot site Lower Silesian Oncology, Pulmonology and Hematology Center (Wroclaw, Poland).
-
- Letter of Acknowledgement from pilot site Lung Cancer Network Luxembourg
Download Document (PDF file, 196 kB)
- Letter of Acknowledgement from pilot site Lung Cancer Network Luxembourg
Report on Setting up Lung CCCNs
Download Document (PDF file, 6.831 kB)
Background and previous work
JA CanCon
- Cancon Guide, Chapter 5: Integrated cancer control: the case for comprehensive cancer care networks (CCCN) (PDF file, 210 kB)
- CANCON JA: Policy Paper on National Cancer Control Programmes (NCCPs)/Cancer Documents in Europe (PDF file, 80 kB)
- EPAAC JA: European Guide for Quality National Cancer Control Programmes (PDF file, 1.1 MB)
- EPAAC JA: Chapter 9 in the book: Boosting Innovation and Cooperation in European Cancer Control: “From ‘on paper’ to ‘into action’: development of National Cancer Control Programmes in the EU” (PDF file, 3.2 MB)
- EPAAC JA: National Cancer Control Programmes: Analysis of Primary Data from Questionnaires: FINAL PRELIMINARY REPORT (PDF file, 1.4 MB)
- EPAAC JA: The development of European Guide for Quality National Cancer Control Programmes (PDF file, 190 kB)
JA iPAAC
- Report on the basis of the analysis of data from the survey on National Cancer Control Programmes/Cancer documents in EU (PDF file, 1.8 MB)
- Report on the inclusion of Patient Pathways, Quality Indicators, PROMS, and the implementation of Comprehensive Cancer Care Networks in the updates of National Cancer Control Programmes (PDF file, 420 kB)
- Report on the basis of the literature review and terminological assessment of the terms „Governance/Stewardship in/of Cancer Care“ (PDF file, 630 kB)
- Definition and methodical support for patient pathways in Comprehensive Cancer Care Networks (CCCNs)
- Quality Indicators for Colorectal and Pancreatic Cancer to monitor and improve oncological care within Comprehensive Cancer Care Networks (CCCN) (PDF file, 270 kB)
- Implementation of patient-reported outcome assessment in routine cancer care – a systematic review of multicentric programs in Europe (PDF file, 460 kB)
- Framework for the implementation of Patient Reported Outcome Measures (PROMs) in routine cancer care (PDF file, 140 kB)
- Implementation of Comprehensive Cancer Care Networks
-
- Set of Standards for CCCN (PDF file, 470 kB)
- Set of Standards for Colorectal and Pancreatic Cancer Care (PDF file, 360 kB)
- Supporting Document for the Set of Standards for CCCNs (PDF file, 90 kB)
- European Framework for the certification of CCCNs in the course of iPAAC (PDF file, 200 kB)
-
- Corresponding 1pager from Roadmap guide
-
- Framework for the Certification and Designation of Comprehensive Cancer Care Networks (CCCN)
- Report on the Pilot Certifications of Comprehensive Cancer Care Networks (CCCN)
- Evaluation of Oncological Care in CCCNs Through Tumour-Specific Quality Indicators Derived with the iET-QI
- Implementing Patient-Reported Outcome Measures in Cancer Care
- Evaluation of Oncological Care in CCCNs Through Tumour-Specific Quality Indicators Derived with the iET-QI
- Quality cycle oncology: evidence-based guidelines, certified centres and evaluation of results via clinical cancer registries and the certification system
- Standards for the Implementation of Comprehensive Cancer Care Networks (CCCNs)
-
